Biological condensates are membrane-less organelles that organize material inside biological cells. It is now understood that the often form by the physical process of liquid-liquid phase separation, but how cells regulate this process is still elusive. We use concepts from theoretical physics to predict the behavior of biological condensates to understand their behavior. For instance, we investigate how cells can control droplets by chemical reactions.
Physics of droplet regulation in biological cells
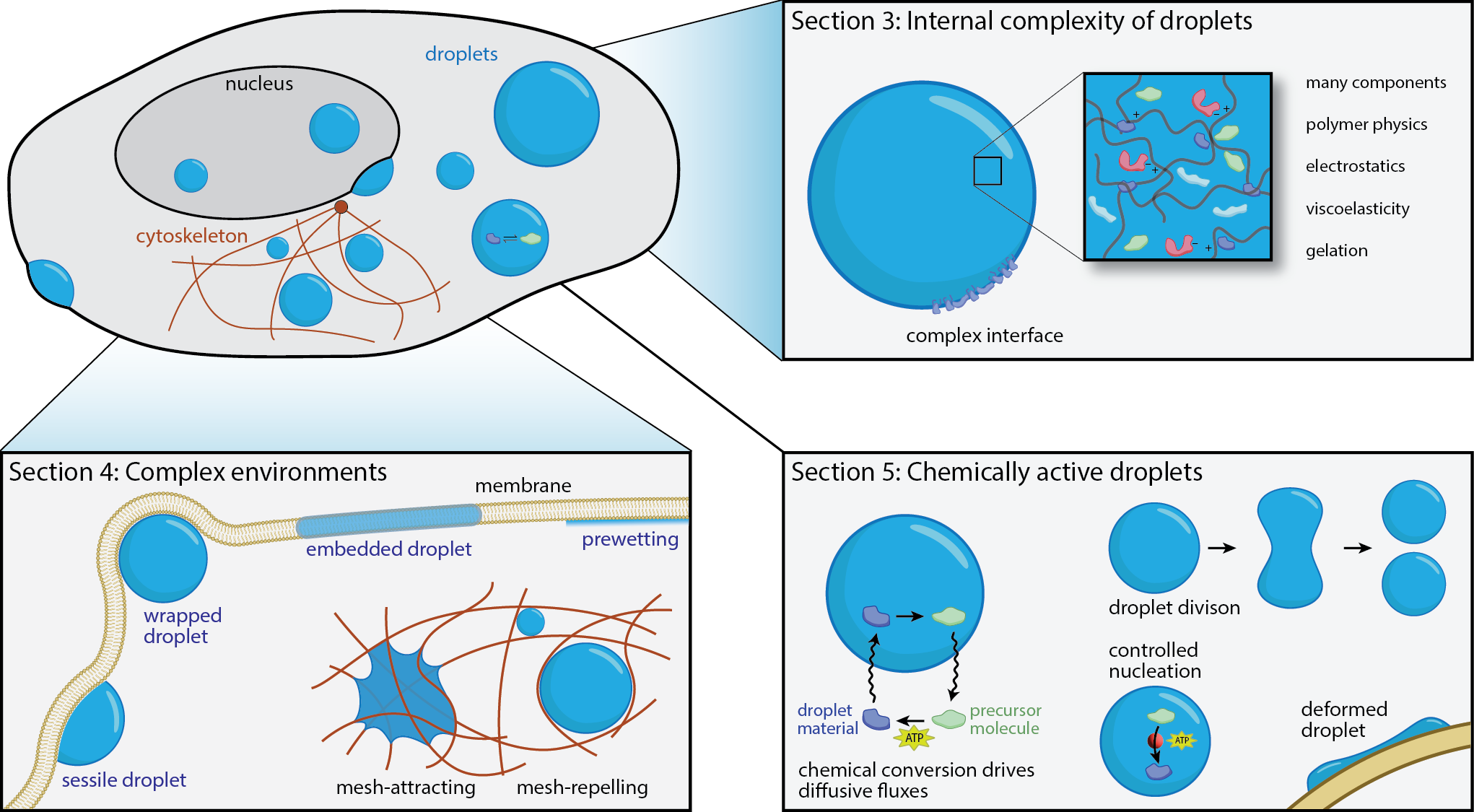
Droplet formation has emerged as an essential concept for the spatiotemporal organization of biomolecules in cells. However, classical descriptions of droplet dynamics based on passive liquid–liquid phase separation cannot capture the complex situation inside cells. Our recent review discusses three distinct aspects that are crucial in cells: (i) biomolecules are diverse and individually complex, implying that cellular droplets possess complex internal behavior, e.g. in terms of their material properties; (ii) the cellular environment contains many solid-like structures that droplets can wet; (iii) cells are alive and use fuel to drive processes out of equilibrium. We illustrate how these principles control droplet nucleation, growth, position, and count to unveil possible regulatory mechanisms in biological cells.
Schematic illustration of densely packed molecules in a cell.
Phase separation in multicomponent fluids
Biological cells are incredibly complex and comprise thousands of molecules that diffuse and interact. To exhibit robust function, cells must thus separate molecules spatially into different compartments. Biomolecular condensates formed by phase separation are an elegant solution to this problem. Here, the interactions between biomolecules create dynamic compartments without requiring energy input. However, cells contain many different condensates simultaneously, and many condensates comprise numerous different biomolecules. This situation is qualitatively different from the ordinary phase separation of a few components we typically study in physics. To understand the qualitative effects that emerge from the interplay of many components, we investigate theoretical models of such multi-component fluids. One important finding is that evolution might have optimized the interactions between biomolecules to form the right condensates robustly.
Qiang et al., Scaling of phase count in multicomponent liquids, Phys. Rev. Res. 2025
Luo et al., Condensate size control by net charge, ACS Macro Lett. 2025
Zwicker, The intertwined physics of active chemical reactions and phase separation, Curr. Opin. Colloid Interface Sci. 2022 [free version on arxiv]
Crossover interference in meiotic recombination
Recombination during meiosis is a crucial process to ensure genetic diversity. It turns out that the locations where the maternal and paternal DNA are crossed over are determined by tiny protein accumulations called foci. These foci are numerous at the beginning of the process, but become fewer and larger at the end. This coarsening process is surprisingly similar to Ostwald ripening and explains a phenomenon called “crossover interference”, which ensures that crossovers are not close to each other.
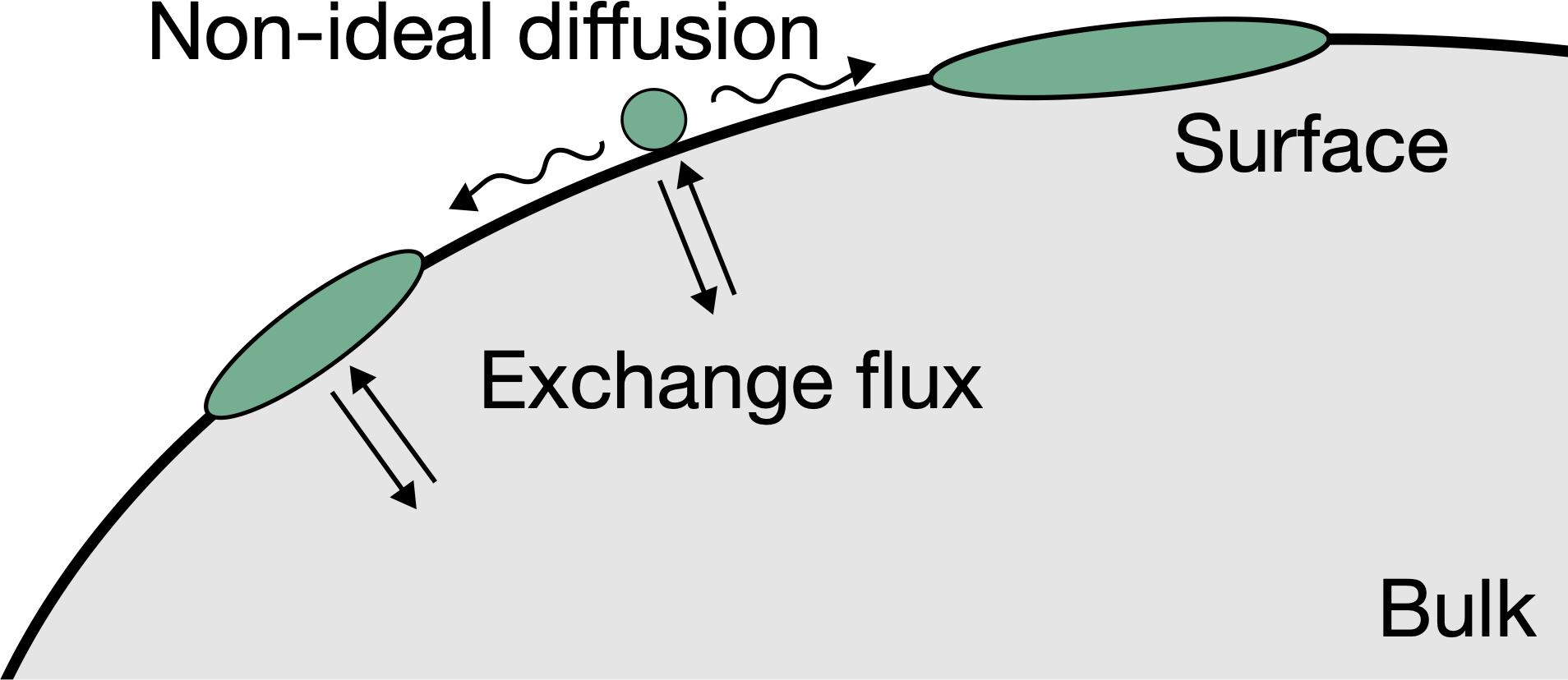
Surface condensates
Biological membranes often exhibit heterogeneous protein patterns, which cells control. Some patterns can be described as surface condensates, formed by physical interactions between constituents, which can typically exchange with the bulk cytosol. We study this situation theoretically by analyzing thermodynamic and kinetic models to explain experimental data of relevant biological processes, including polarity spot formation in budding yeast and polarity establishment in C. elegans.
Mechanisms of active regulation of biomolecular condensates
Cells need to regulate the size of their biomolecular condensates, e.g., to allow multiple condensates to exist for a long time. It is difficult to control the size of droplets since their surface tension implies that large droplets grow at the expense of smaller ones. This culminates in a state where only a single droplet remains. We propose that chemical reactions that affect the droplet material can drive a compensating flux from larger to smaller droplets. These chemical reactions will consume fuel like ATP. Their reaction rate can be used to control droplet size. Based on these ideas, we propose two qualitatively different models for size regulation: In enrichment-inhibition, the reaction is catalyzed inside the droplet to remove droplet material from larger droplets. Conversely, in localization-induction, droplet material is created at catalytic sites to form droplets around them. We find evidence for both models in nature.
Kusters et al., Size control guidelines for chemically active droplets, arXiv
Fries et al., Chemically active droplets in crowded environments, PRE (in press), arXiv
Söding et al., Mechanisms of active regulation of biomolecular condensates, Trends Cell Biol. 2020
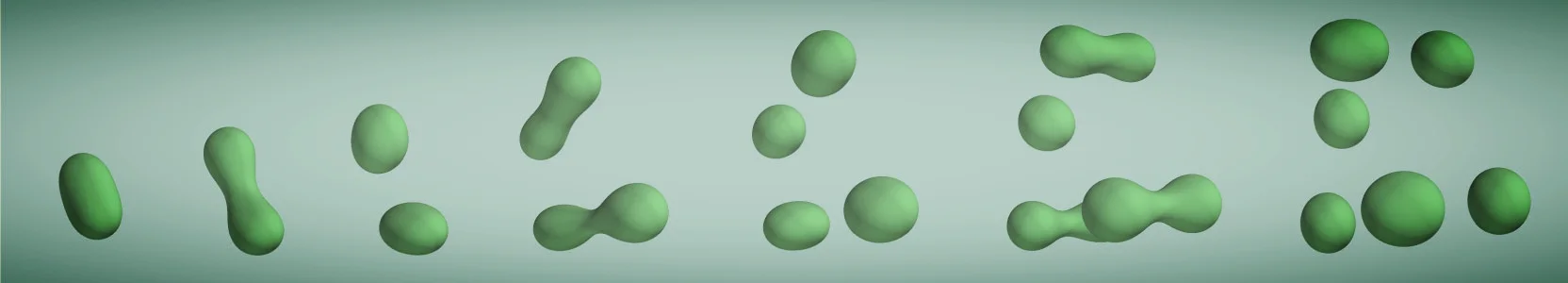
Spontaneously dividing active droplets as a model for protocells
The first lifeforms on earth must have been simple enough to emerge spontaneously, but they also needed to divide and propagate, such that natural selection and evolution could lead to more complex lifeforms. So far, the physical properties of such early cells are unclear, but it has been proposed almost a century ago that they could have been liquid-like droplets. We showed that such simple liquid droplets can indeed divide spontaneously if the chemical reaction that builds the droplet material from precursors is driven by an external energy input, like sun light or heat from thermal vents. Here, the chemical reaction of these active droplets plays the role of a prebiotic metabolism.
Centrosomes described as active droplets
Centrosomes are small organelles present in all animal cells. They are important for organizing the mitotic spindle, which segregates the DNA during cell division. Cancer cells often contain more than two centrosomes, which impairs normal cell division. It is thus important to understand the assembly of centrosomes in order to tackle failures in their formation and function.
The centrosome is a dynamic aggregate, which forms and dissolves in synchrony with the cell cycle. Beside reactions between and diffusion of centrosome components, aspects of non-equilibrium thermodynamics have to be considered to describe the observed behavior. In fact, we developed a physical description of centrosomes as autocatalytic droplets, whose formation is controlled by chemical reactions. This project was carried out in close collaboration with the group of Tony Hyman at the MPI of Molecular Cell Biology and Genetics.
Recently, we have also shown that the same model also explains why centrioles are always found at the center of the centrosome: The chemical reactions in the centrosome and at the centriole surface induce compositional fluxes, which lead to the effective centering of the centrioles.